
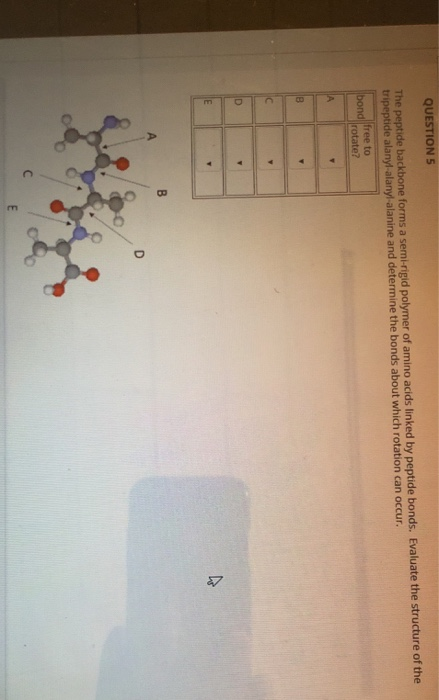
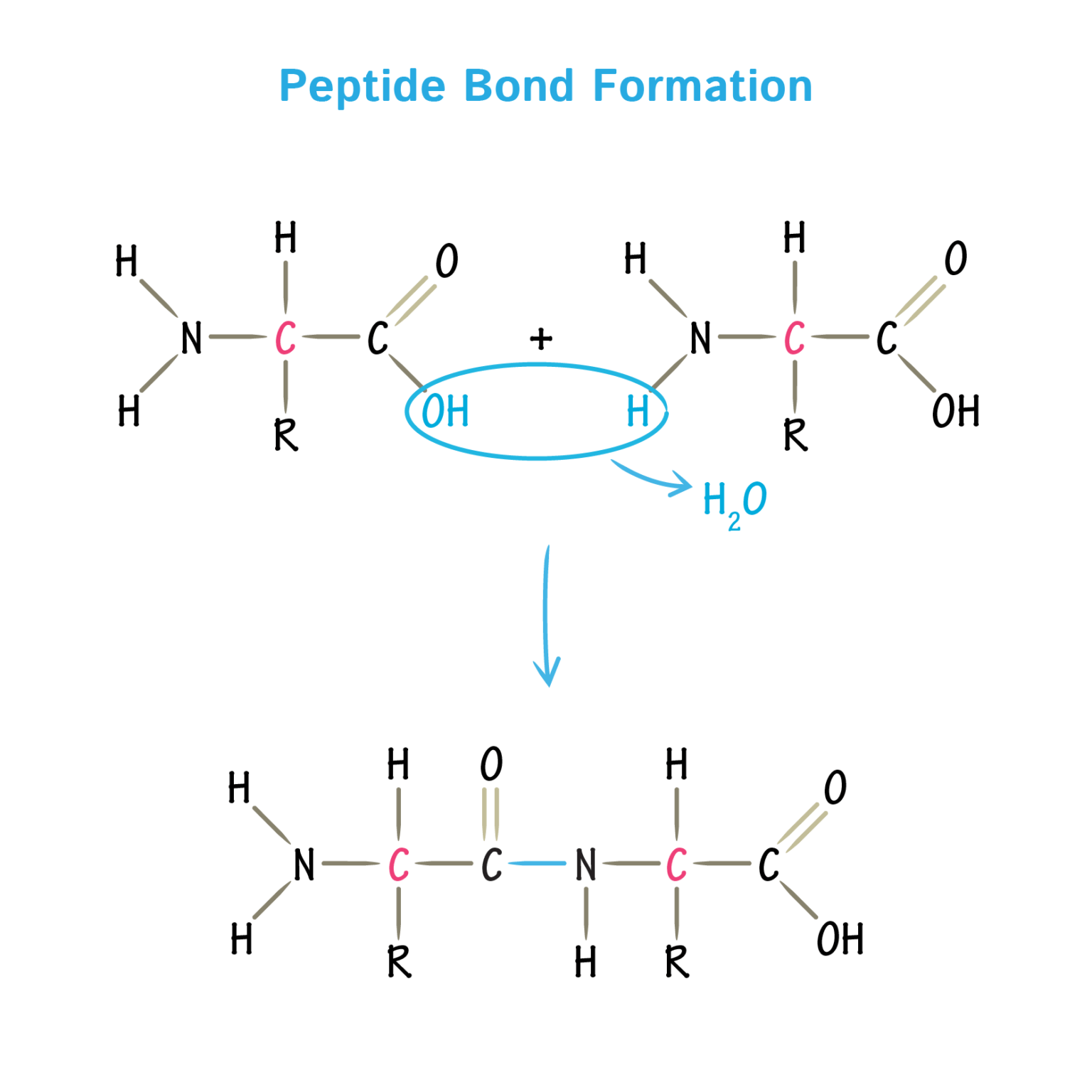
The majority of alpha-helices in globular proteins are curved or distorted somewhat compared with the standard Pauling-Corey model. All the amino acids have negative phi and psi angles, typical values being -60 degrees and -50 degrees, respectively.Side chains point outward from helix axis and are generally oriented towards its amino-terminal end. The peptide planes are roughly parallel with the helix axis and the dipoles within the helix are aligned, ie all C=O groups point in the same direction and all N-H groups point the other way.This gives a very regular, stable arrangement. Every mainchain C=O and N-H group is hydrogen-bonded to a peptide bond 4 residues away (ie O(i) to N(i+4)).The separation of residues along the helix axis is 5.4/3.6 or 1.5 Angstroms, ie the alpha-helix has a rise per residue of 1.5 Angstroms. Alpha-helices have 3.6 amino acid residues per turn, ie a helix 36 amino acids long would form 10 turns. The structure repeats itself every 5.4 Angstroms along the helix axis, ie we say that the alpha-helix has a pitch of 5.4 Angstroms.For each hand the thumbs indicate the direction of translation and the fingers indicate the direction of rotation. An easy way to remember this is to hold both your hands in front of you with your thumbs pointing up and your fingers curled towards you. The figure below shows how a right-handed helix differs from a left-handed one. The most simple and elegant arrangement is a right-handed spiral conformation known as the 'alpha-helix'. Pauling and Corey twisted models of polypeptides around to find ways of getting theīackbone into regular conformations which would agree with alpha-keratin fibre diffraction data. In rare cases omega = 0 degrees for a cis peptide bond which, as stated above, usually involves proline.ĭevelopment of a Model for Alpha-Helix Structure. The planarity of the peptide bond restricts omega to 180 degrees in very nearly all of the main chain peptide bonds. The figure below shows the three main chain torsion angles of a polypeptide.
Peptide Torsion Angles and Secondary Structure Peptide Torsion Angles and Secondary Structure Back to Index


 0 kommentar(er)
0 kommentar(er)
